VIDAS® Fertility panel
Investigation and treatment of infertility
The VIDAS® Fertility panel includes 7 fully automated hormone tests for the quantitative measurement of luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, prolactin, progesterone, testosterone and human chorionic gonadotropin (hCG).
- Clinically validated estradiol and progesterone assays
- Convenient solution for fertility centers to perform on-site testing
The VIDAS® Fertility panel provides key diagnostic tools for the investigation of reproductive hormone dysfunctions such as precocious and delayed puberty, amenorrhea, hirsutism, hyperprolactinemia, perimenopause and menopause, hypogonadism, gynecomastia and azoospermia. In assisted reproductive technologies (ART), correct diagnosis of the cause of infertility is a pre-requisite to successful treatment. In addition, our hormone tests are essential monitoring tools in IVF protocols. They contribute to the optimization of individual patient treatment for increased chances of success.
Hormone testing in ART
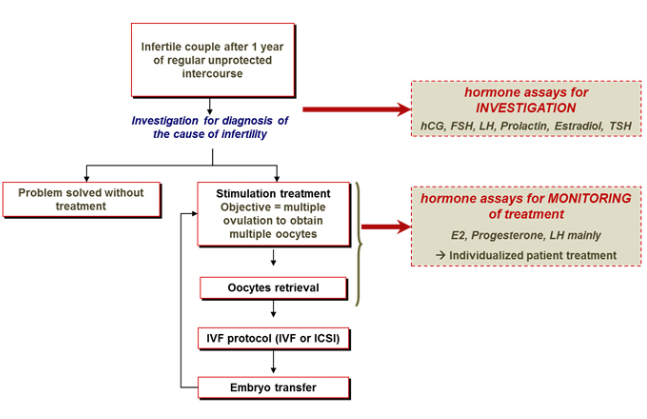
Hormone measurements are essential to personalize treatment of infertility and monitor the progress of ovarian stimulation.
Before treatment, they allow to identify the type of ovulation disorder and know the patient’s ovarian reserve.
During treatment, they are used to check compliance, optimize drug doses, and avoid futile embryo replacements and severe complications.
Hormone assays in the diagnosis and treatment of infertility
Precise and sensitive tools
Clinical evaluation of the VIDAS® Estradiol and VIDAS® Progesterone assays has confirmed their reliability for use in evaluating natural cycles and monitoring ovarian superovulation1:
- Accuracy in the low concentration range to confirm pituitary suppression after GnRH-agonist therapy
- Strong correlation between serum estradiol measured by VIDAS® Estradiol and follicular measurements by ultrasonography during gonadotropin treatment with both GnRH analogue protocol
Testing made simple for fertility clinics
Easy-to-use solution:
- No need for water
- All-inclusive reagent kits with ready-to-use reagents
- Just Load & Go
- No need for complex training
- Easy maintenance
- Robust instrument: MTBF > 700 days
Enables on-site testing for key advantages:
- Rapid results
- Faster adaptation of stimulation treatment
- Cost-effectiveness
Fertility, thyroid and serology testing can be performed on the same instrument:
- Broad VIDAS® test menu including HIV, hepatitis, toxoplasmosis, rubella, CMV, TSH, FT3, FT4 and Anti-TPO
Reference
1. E. Anckaert et al. Clinical Validation of a Fully Automated 17β-Estradiol and Progesterone Assay (VIDAS®) for Use in Monitoring Assisted Reproduction Treatment. Clin Chem Lab Med 2002; 40(8):824-831.
TECHNICAL DETAILS
|
|
VIDAS® Estradiol II |
VIDAS® FSH |
VIDAS® HCG |
VIDAS® LH |
|
Reference |
3- 431 |
30 407 |
30 405 |
30 406 |
|
Tests / kit |
60 |
60 |
60 |
60 |
|
Sample type |
Human serum or plasma |
Human serum or plasma |
Human serum or plasma |
Human serum or plasma |
|
Sample volume |
200µL |
200µL |
100µL |
200µL |
|
|
VIDAS® Prolactin |
VIDAS® Progesterone |
VIDAS® Testosterone |
|
Reference |
30 410 |
30 409 |
30 418 |
|
Test / kit |
60 |
60 |
30 |
|
Sample type |
Human serum or plasma |
Human serum or plasma |
Human serum or plasma (on heparin) |
|
Sample volume |
200 µL |
200 µL |
200 µL |
|
VIDAS® Testosterone II |
|
|
Reference |
414 320 |
|
Tests / kit |
30 |
|
Sample type |
Human serum or plasma |
|
Sample type |
100 µL |
RESOURCES
Useful Links
European Society of Human Reproduction and Embryology (ESHRE)
American Society for Reproductive Medicine (ASRM)
Poster
- Product: VIDAS® Fertility panel
- SKU:
- Availability: In Stock
Tags: VIDAS® Fertility panel